Decoding the Periodic Desk: A Deep Dive into Parts, Their Properties, and Valency
Associated Articles: Decoding the Periodic Desk: A Deep Dive into Parts, Their Properties, and Valency
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Decoding the Periodic Desk: A Deep Dive into Parts, Their Properties, and Valency. Let’s weave attention-grabbing data and supply contemporary views to the readers.
Desk of Content material
Decoding the Periodic Desk: A Deep Dive into Parts, Their Properties, and Valency

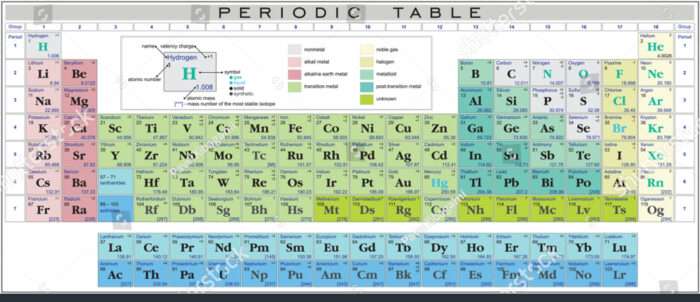
The periodic desk of parts, a cornerstone of chemistry, is greater than only a neatly organized record of atoms. It is a dynamic illustration of the basic constructing blocks of matter, showcasing their properties, relationships, and predictive energy. At its coronary heart lies the idea of valency, an important issue figuring out how parts work together and type compounds. This text will discover the periodic desk, specializing in the connection between elemental properties and valency, delving into the intricacies of electron configuration and its influence on chemical conduct.
The Group of the Periodic Desk:
The desk’s group is not arbitrary; it displays the underlying construction of atoms. Parts are organized so as of accelerating atomic quantity (the variety of protons within the nucleus), which dictates their chemical id. The desk is additional structured into durations (horizontal rows) and teams (vertical columns).
-
Intervals: Every interval represents a principal power stage (shell) in an atom. Parts inside the identical interval have the identical variety of electron shells. As you progress throughout a interval, the variety of electrons within the outermost shell (valence shell) will increase, resulting in a gradual change in properties.
-
Teams: Parts inside the identical group share comparable chemical properties as a result of they’ve the identical variety of valence electrons. These valence electrons are the important thing gamers in chemical bonding, figuring out a component’s reactivity and the sorts of compounds it might type. Group 1 parts (alkali metals) all have one valence electron, making them extremely reactive. Group 18 parts (noble gases) have a full valence shell, making them exceptionally unreactive.
Electron Configuration and Valency:
The important thing to understanding valency lies in an atom’s electron configuration. Electrons occupy particular power ranges and sublevels inside an atom, described by quantum numbers. The outermost shell, the valence shell, accommodates the valence electrons. The variety of valence electrons straight determines a component’s valency.
Valency refers back to the combining capability of a component, primarily the variety of electrons an atom can achieve, lose, or share to realize a secure electron configuration, normally resembling that of a noble fuel (a full octet, aside from hydrogen and helium which obtain stability with two electrons).
-
Electropositive Parts (Metals): Metals are inclined to lose valence electrons to realize a secure electron configuration, forming positively charged ions (cations). Their valency is normally constructive and corresponds to the variety of electrons misplaced. For instance, sodium (Na) in Group 1 has one valence electron and a valency of +1. Magnesium (Mg) in Group 2 has two valence electrons and a valency of +2. Transition metals, nonetheless, can exhibit variable valency because of the involvement of electrons from each the outermost and penultimate shells. Iron (Fe), for example, can have a valency of +2 or +3.
-
Electronegative Parts (Non-metals): Non-metals have a tendency to realize electrons to realize a secure electron configuration, forming negatively charged ions (anions). Their valency is normally unfavourable and corresponds to the variety of electrons gained. Chlorine (Cl) in Group 17 has seven valence electrons and a valency of -1, needing yet one more electron to finish its octet. Oxygen (O) in Group 16 has six valence electrons and a valency of -2, needing two extra electrons.
-
Covalent Bonding: Some parts, significantly non-metals, obtain secure electron configurations by sharing electrons quite than fully transferring them. One of these bonding known as covalent bonding. The valency in covalent compounds represents the variety of covalent bonds an atom can type. Carbon (C), for instance, has 4 valence electrons and a valency of 4, forming 4 covalent bonds.
Predicting Valency from the Periodic Desk:
The periodic desk supplies a robust device for predicting the valency of parts. Whereas there are exceptions, the next generalizations may be made:
- Group 1 (Alkali Metals): Valency of +1
- Group 2 (Alkaline Earth Metals): Valency of +2
- Group 13 (Boron Group): Valency of +3 (although some exceptions exist)
- Group 14 (Carbon Group): Valency of +4 or -4 (relying on the electronegativity of the bonding accomplice)
- Group 15 (Pnictogens): Valency of -3 or +5 (relying on the bonding accomplice)
- Group 16 (Chalcogens): Valency of -2
- Group 17 (Halogens): Valency of -1
- Group 18 (Noble Gases): Valency of 0 (typically inert)
Exceptions and Problems:
Whereas the periodic desk supplies a worthwhile framework for predicting valency, it is essential to acknowledge exceptions. Transition metals, as talked about earlier, exhibit variable valency because of the advanced interaction of d-electrons. Some parts can exhibit a number of valencies relying on the response situations and the character of the bonding accomplice. For instance, copper (Cu) can have a valency of +1 or +2. Lead (Pb) can have a valency of +2 or +4. Understanding these exceptions usually requires a deeper understanding of the digital construction and bonding rules concerned.
Functions of Valency:
Understanding valency is essential in quite a few areas of chemistry:
-
Formulation Writing: Valency is important for writing the right chemical formulation of compounds. The valencies of the constituent parts decide the ratio during which they mix. For instance, the valency of sodium is +1 and the valency of chlorine is -1, resulting in the system NaCl (sodium chloride).
-
Chemical Reactions: Valency dictates how parts react with one another. Parts with excessive valencies are typically extra reactive than these with decrease valencies.
-
Predicting Properties: The valency of a component will help predict sure properties of a compound, reminiscent of its melting level, boiling level, and solubility.
-
Structural Chemistry: Valency performs a essential function in figuring out the three-dimensional construction of molecules.
-
Industrial Functions: Valency is essential in varied industrial processes, such because the synthesis of latest supplies and the design of catalysts.
Conclusion:
The periodic desk, with its inherent group based mostly on atomic construction, supplies a robust framework for understanding the properties of parts, significantly their valency. Valency, decided by the variety of valence electrons, dictates how parts work together, type compounds, and exhibit their attribute chemical conduct. Whereas there are exceptions and complexities, understanding valency stays a basic idea for anybody searching for to grasp the intricacies of chemistry. The power to foretell and perceive valency permits chemists to synthesize new supplies, design efficient reactions, and unravel the basic rules governing the conduct of matter. It is a testomony to the elegant simplicity and profound energy of the periodic desk – a chart that continues to form our understanding of the world round us.







Closure
Thus, we hope this text has offered worthwhile insights into Decoding the Periodic Desk: A Deep Dive into Parts, Their Properties, and Valency. We hope you discover this text informative and useful. See you in our subsequent article!