Decoding the Periodic Desk: A Deep Dive into the Atomic Mass Chart
Associated Articles: Decoding the Periodic Desk: A Deep Dive into the Atomic Mass Chart
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Decoding the Periodic Desk: A Deep Dive into the Atomic Mass Chart. Let’s weave fascinating data and provide recent views to the readers.
Desk of Content material
Decoding the Periodic Desk: A Deep Dive into the Atomic Mass Chart

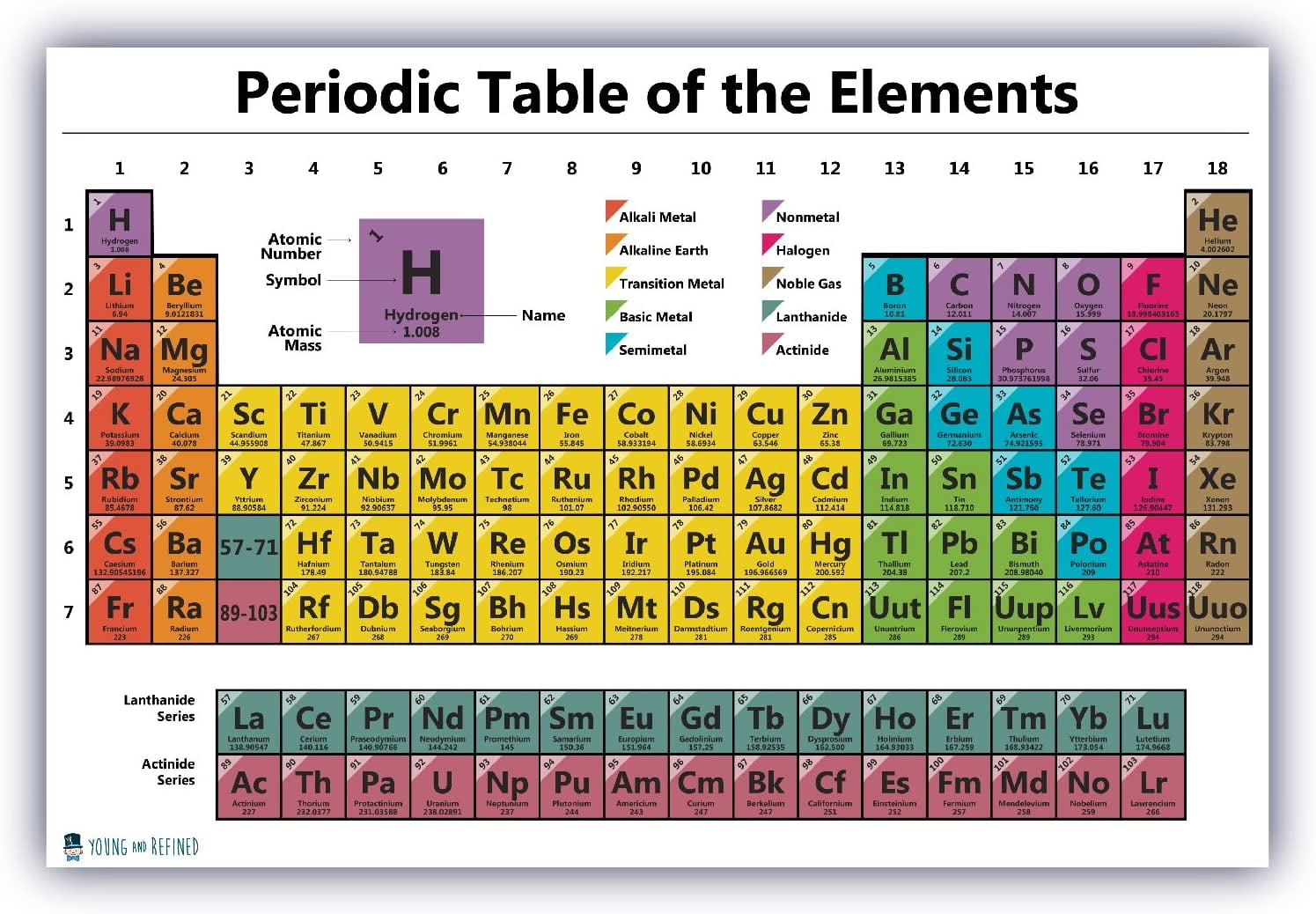
The periodic desk, a cornerstone of chemistry, organizes components based mostly on their atomic quantity and recurring chemical properties. Nonetheless, an important piece of knowledge related to every component is its atomic mass, a worth reflecting the common mass of a component’s atoms, contemplating the relative abundance of its isotopes. This text delves into the intricacies of the atomic mass chart, exploring its significance, the way it’s decided, its functions, and the refined complexities inherent in its interpretation.
Understanding Atomic Mass: Past the Easy Common
The atomic mass of a component is not merely the mass of a single atom. Atoms of the identical component can exist as isotopes, differing within the variety of neutrons of their nuclei whereas retaining the identical variety of protons. This distinction in neutron rely results in variations in atomic mass. For instance, carbon has two main isotopes: carbon-12 (¹²C) with six protons and 6 neutrons, and carbon-13 (¹³C) with six protons and 7 neutrons. The atomic mass listed on the periodic desk is a weighted common of the lots of all naturally occurring isotopes of a component, weighted by their relative abundances.
The formulation for calculating the common atomic mass is:
*Atomic Mass = Σ (mass of isotope fractional abundance of isotope)**
The place the summation (Σ) is taken over all naturally occurring isotopes of the component. The fractional abundance represents the proportion of every isotope present in nature. As an illustration, carbon-12 accounts for about 98.93% of naturally occurring carbon, whereas carbon-13 accounts for about 1.07%. This weighted common provides carbon an atomic mass of roughly 12.011 atomic mass models (amu).
The Atomic Mass Unit (amu): A Commonplace of Measurement
The atomic mass unit (amu), also referred to as the dalton (Da), is a typical unit used to specific the mass of atoms and molecules. It is outlined as one-twelfth the mass of a single carbon-12 atom. Because of this one amu is roughly 1.66 x 10⁻²⁴ grams. Utilizing amu permits for handy comparisons of atomic lots with out coping with extraordinarily small numbers.
Sources of Knowledge for Atomic Mass Willpower
The atomic lots introduced within the periodic desk aren’t arbitrary figures; they’re meticulously decided by way of varied subtle strategies. Mass spectrometry is a cornerstone approach for isotope evaluation. This technique includes ionizing atoms, accelerating them by way of a magnetic discipline, and separating them based mostly on their mass-to-charge ratio. The relative abundance of every isotope is set by measuring the depth of the respective ion beams.
Different strategies, reminiscent of nuclear magnetic resonance (NMR) spectroscopy and X-ray diffraction, also can present complementary information on isotopic ratios and contribute to the refinement of atomic mass values. These information are repeatedly collected and analyzed by organizations just like the Worldwide Union of Pure and Utilized Chemistry (IUPAC) and the Worldwide Union of Pure and Utilized Physics (IUPAP), which often replace the usual atomic weights.
Variations and Uncertainties in Atomic Mass Values
It is vital to notice that the atomic lots listed on the periodic desk are sometimes introduced with uncertainties. These uncertainties replicate the inherent variability within the isotopic composition of components discovered in several places on Earth. Geological processes, reminiscent of radioactive decay and fractionation, can affect the relative abundances of isotopes. For instance, the isotopic composition of a component in a volcanic rock would possibly differ from that in a sedimentary rock.
Moreover, the atomic mass of a component can range relying on the supply of the pattern. That is significantly true for components with a number of isotopes and important isotopic fractionation. This variation is usually small, however it may be important in sure analytical functions requiring excessive precision. The uncertainties related to atomic mass values are mirrored within the variety of decimal locations reported.
Purposes of the Atomic Mass Chart
The atomic mass chart, embedded inside the periodic desk, is indispensable throughout quite a few scientific disciplines. Its functions embrace:
-
Stoichiometry and Chemical Calculations: Atomic mass is essential for calculating the molar mass of compounds, which is important for performing stoichiometric calculations in chemical reactions. Understanding the mass relationships between reactants and merchandise is key to quantitative chemistry.
-
Nuclear Chemistry and Physics: In nuclear chemistry, atomic mass performs a significant position in understanding nuclear reactions, radioactive decay, and the steadiness of isotopes. The variations in atomic lots between isotopes are instantly associated to the variety of neutrons and contribute to the understanding of nuclear forces.
-
Analytical Chemistry: Atomic mass is crucial in varied analytical strategies, together with mass spectrometry, which is used for figuring out and quantifying completely different substances in a pattern. The exact willpower of atomic lots helps in figuring out unknown compounds and analyzing isotopic ratios.
-
Geochemistry and Cosmochemistry: Isotopic ratios of components, linked to their atomic lots, present helpful insights into geological processes, the age of rocks, and the origins of the photo voltaic system. Variations in isotopic abundances can reveal details about previous environmental circumstances and planetary formation.
-
Biochemistry and Medication: Isotopic labeling utilizing isotopes with completely different atomic lots is a strong software in biochemistry and medication. This system permits researchers to trace the motion of molecules inside organic programs and examine metabolic pathways. Isotopes like ¹³C and ¹⁵N are regularly utilized in metabolic research.
Past the Common: Isotopic Abundance and its Significance
Whereas the common atomic mass is a helpful worth, it is important to grasp that it represents a mean. In lots of functions, data of the precise isotopic composition of a pattern is essential. As an illustration, in forensic science, analyzing the isotopic ratios of sure components in a pattern may help to hint its origin. Equally, in environmental research, isotopic ratios can be utilized to trace pollution and decide their sources.
The examine of isotopic variations, sometimes called isotopic geochemistry or isotopic hydrology, supplies highly effective instruments for understanding varied pure processes. These variations can be utilized to hint the motion of water, determine the sources of air pollution, and decide the age of supplies.
Conclusion: A Basis of Chemical Understanding
The atomic mass chart, an integral a part of the periodic desk, supplies a elementary piece of details about every component. Whereas the common atomic mass affords a handy illustration, it is vital to understand the underlying complexities of isotopic variations and their significance in varied scientific disciplines. The continual refinement of atomic mass values by way of superior analytical strategies underscores the continuing quest for precision and deeper understanding of the basic constructing blocks of matter. The atomic mass, due to this fact, just isn’t merely a quantity on a desk; it is a window into the wealthy isotopic range of components and a key to unlocking numerous scientific discoveries.


:max_bytes(150000):strip_icc()/PeriodicTable-56a12c983df78cf772682271.png)



Closure
Thus, we hope this text has offered helpful insights into Decoding the Periodic Desk: A Deep Dive into the Atomic Mass Chart. We admire your consideration to our article. See you in our subsequent article!