Decoding the pH Chart: A Complete Information to the Acidity and Alkalinity of Frequent Liquids
Associated Articles: Decoding the pH Chart: A Complete Information to the Acidity and Alkalinity of Frequent Liquids
Introduction
On this auspicious event, we’re delighted to delve into the intriguing matter associated to Decoding the pH Chart: A Complete Information to the Acidity and Alkalinity of Frequent Liquids. Let’s weave attention-grabbing data and supply recent views to the readers.
Desk of Content material
Decoding the pH Chart: A Complete Information to the Acidity and Alkalinity of Frequent Liquids
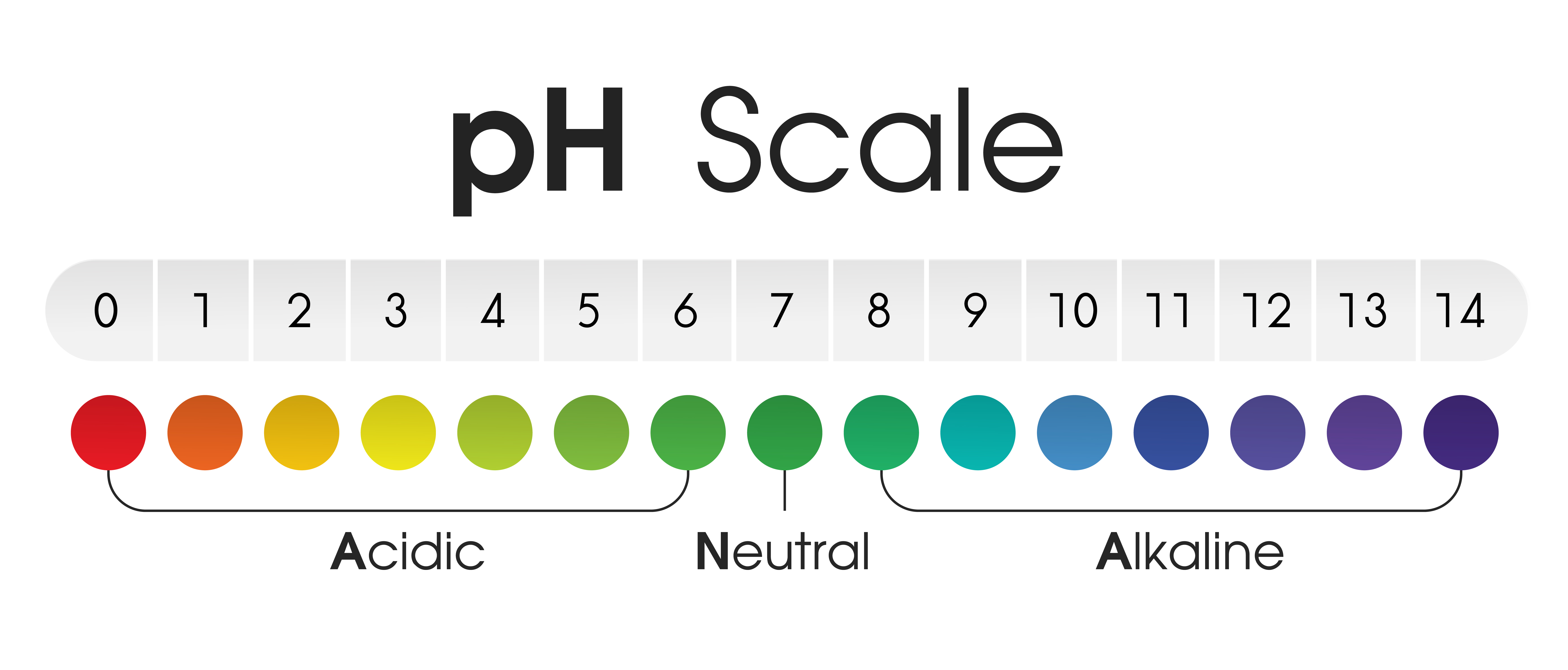
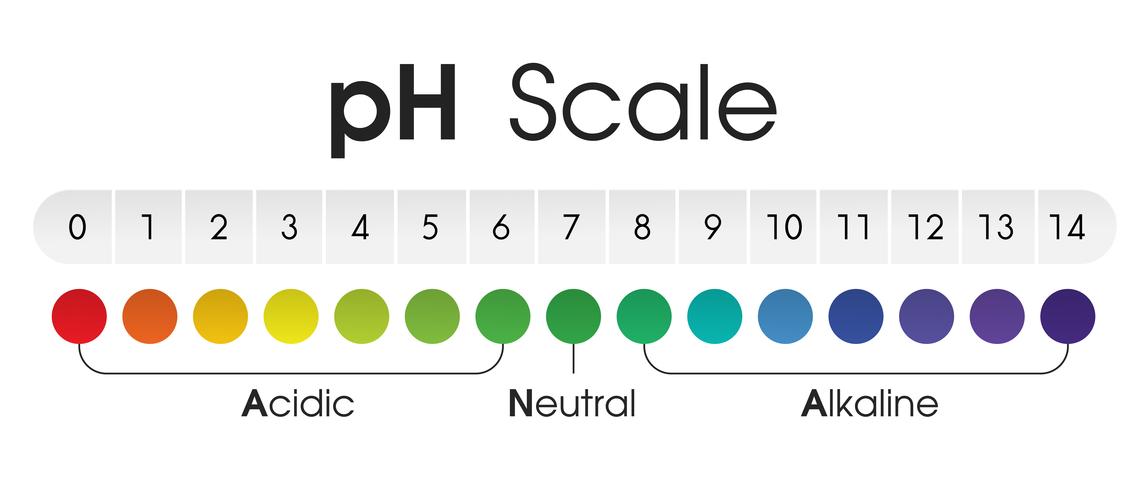
The pH scale, a logarithmic scale starting from 0 to 14, is a vital device for understanding the acidity or alkalinity of an answer. A pH of seven represents neutrality, with values beneath 7 indicating acidity and values above 7 indicating alkalinity. This seemingly easy scale has profound implications throughout numerous fields, from chemistry and biology to agriculture and environmental science. Understanding the pH of frequent liquids is important for quite a few purposes, from sustaining wholesome soil for gardening to making sure the protection of commercial processes. This text delves into the pH chart of frequent liquids, explaining the components influencing pH and offering sensible examples of its significance.
The Fundamentals of pH:
The pH of an answer is decided by the focus of hydrogen ions (H+) current. A better focus of H+ ions ends in a decrease pH (extra acidic), whereas a decrease focus of H+ ions ends in a better pH (extra alkaline). The size is logarithmic, which means every entire quantity change represents a tenfold distinction in H+ ion focus. For example, an answer with a pH of three is ten occasions extra acidic than an answer with a pH of 4, and 100 occasions extra acidic than an answer with a pH of 5.
The pH scale just isn’t linear; the transition from acidic to impartial to alkaline just isn’t uniform. The notion of acidity or alkalinity can also be in a roundabout way proportional to the pH worth. A slight change in pH can have vital penalties, particularly in organic techniques.
Elements Influencing the pH of Liquids:
A number of components contribute to the pH of a liquid:
-
The character of the dissolved substance: Sturdy acids (like hydrochloric acid) and powerful bases (like sodium hydroxide) utterly dissociate in water, leading to a dramatic change in pH. Weak acids and bases solely partially dissociate, resulting in a milder change in pH.
-
Focus: The focus of the dissolved substance instantly impacts the pH. A better focus of an acid will lead to a decrease pH, and vice versa for a base.
-
Temperature: Temperature can affect the dissociation of acids and bases, thereby affecting the pH. Usually, a rise in temperature can improve the ionization of water, barely decreasing the pH of impartial options.
-
Presence of buffers: Buffers are substances that resist adjustments in pH when small quantities of acid or base are added. They’re essential in sustaining steady pH ranges in organic techniques.
pH Chart of Frequent Liquids:
The next chart gives an approximate pH vary for numerous frequent liquids. It is vital to notice that the precise pH can fluctuate relying on components equivalent to purity, focus, and temperature.
| Liquid | pH Vary | Notes |
|---|---|---|
| Battery Acid | 0-1 | Extremely corrosive sulfuric acid |
| Gastric Acid (Abdomen Acid) | 1.5-3.5 | Extremely acidic, essential for digestion |
| Lemon Juice | 2-3 | Accommodates citric acid |
| Vinegar | 2.5-3.5 | Acetic acid resolution |
| Orange Juice | 3-4 | Accommodates citric and ascorbic acids |
| Cola Drinks | 2.5-3.5 | Phosphoric acid and different acids contribute to acidity |
| Espresso | 4.5-5.5 | Varies relying on brewing methodology and bean kind |
| Black Tea | 4.5-5.5 | Accommodates tannins and different acidic compounds |
| Rainwater | 5.0-6.0 | Barely acidic on account of dissolved carbon dioxide (carbonic acid) |
| Pure Water | 7.0 | Impartial at 25°C |
| Saliva | 6.5-7.5 | Barely acidic to impartial, varies relying on food regimen and well being |
| Blood | 7.35-7.45 | Tightly regulated pH vary essential for correct bodily operate |
| Milk | 6.5-6.8 | Barely acidic |
| Baking Soda Resolution | 8-9 | Alkaline resolution, generally used as an antacid |
| Seawater | 7.5-8.4 | Barely alkaline on account of dissolved salts |
| Family Ammonia | 11-12 | Extremely alkaline, corrosive |
| Drain Cleaner (Lye) | 13-14 | Extraordinarily alkaline, extremely corrosive sodium hydroxide resolution |
Purposes of pH Measurement:
The measurement and understanding of pH are essential in quite a few purposes:
-
Agriculture: Soil pH considerably impacts nutrient availability to crops. Totally different crops thrive in numerous pH ranges. Testing soil pH permits farmers and gardeners to regulate soil situations utilizing amendments like lime (to lift pH) or sulfur (to decrease pH).
-
Aquaculture: Sustaining the proper pH in aquaculture techniques is essential for the well being and survival of aquatic organisms. Variations in pH can stress fish and different aquatic life, making them prone to illnesses.
-
Meals and Beverage Business: pH management is important in meals processing and preservation. Many meals preservation methods depend on controlling pH to inhibit microbial progress. The pH of drinks additionally impacts their style and high quality.
-
Drugs: The pH of bodily fluids, equivalent to blood and urine, is rigorously monitored to diagnose and handle numerous well being situations. Sustaining a correct pH stability is crucial for general well being. Many drugs are formulated to have particular pH ranges for optimum absorption and efficacy.
-
Environmental Monitoring: Measuring the pH of water our bodies is essential for assessing water high quality and figuring out air pollution sources. Acid rain, for instance, considerably lowers the pH of lakes and rivers, harming aquatic life.
-
Industrial Processes: Many industrial processes require exact pH management. For instance, the manufacturing of prescription drugs, chemical compounds, and paper typically entails cautious pH changes to make sure product high quality and security.
Measuring pH:
pH will be measured utilizing numerous strategies:
-
pH indicators: These are substances that change coloration relying on the pH of the answer. Litmus paper is a standard instance. Whereas handy for a tough estimate, they lack precision.
-
pH meters: These digital gadgets present a exact measurement of pH. They’re broadly utilized in laboratories and industrial settings.
-
pH take a look at strips: These are handy and comparatively cheap alternate options to pH meters, providing a semi-quantitative measure of pH.
Conclusion:
The pH chart of frequent liquids gives a worthwhile overview of the acidity and alkalinity of on a regular basis substances. Understanding the pH scale and its implications is essential throughout numerous fields. From sustaining wholesome soil to making sure the protection of commercial processes and monitoring environmental well being, the flexibility to precisely measure and management pH is crucial for quite a few purposes. The knowledge offered on this article gives a basis for additional exploration into the complexities and significance of pH in numerous points of our lives. Additional analysis into particular purposes and the nuances of pH measurement will improve understanding and contribute to developments in numerous scientific and technological fields.
![[Solved] An aqueous solution with pH = 0 is:](https://sciencenotes.org/wp-content/uploads/2020/09/The-pH-Scale-scaled.jpg)



Closure
Thus, we hope this text has offered worthwhile insights into Decoding the pH Chart: A Complete Information to the Acidity and Alkalinity of Frequent Liquids. We hope you discover this text informative and helpful. See you in our subsequent article!