Decoding the pH Conversion Chart: A Complete Information
Associated Articles: Decoding the pH Conversion Chart: A Complete Information
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Decoding the pH Conversion Chart: A Complete Information. Let’s weave attention-grabbing data and supply recent views to the readers.
Desk of Content material
Decoding the pH Conversion Chart: A Complete Information

The pH scale, a logarithmic measure of hydrogen ion focus, is a elementary idea in chemistry, biology, and quite a few industrial functions. Understanding pH is essential in varied fields, from agriculture and aquaculture to drugs and environmental science. A pH conversion chart, usually introduced as a desk or graph, gives a visible illustration of the connection between pH values, hydrogen ion focus ([H+]), and hydroxide ion focus ([OH-]). This text will delve into the intricacies of pH conversion charts, explaining their development, interpretation, and functions throughout totally different disciplines.
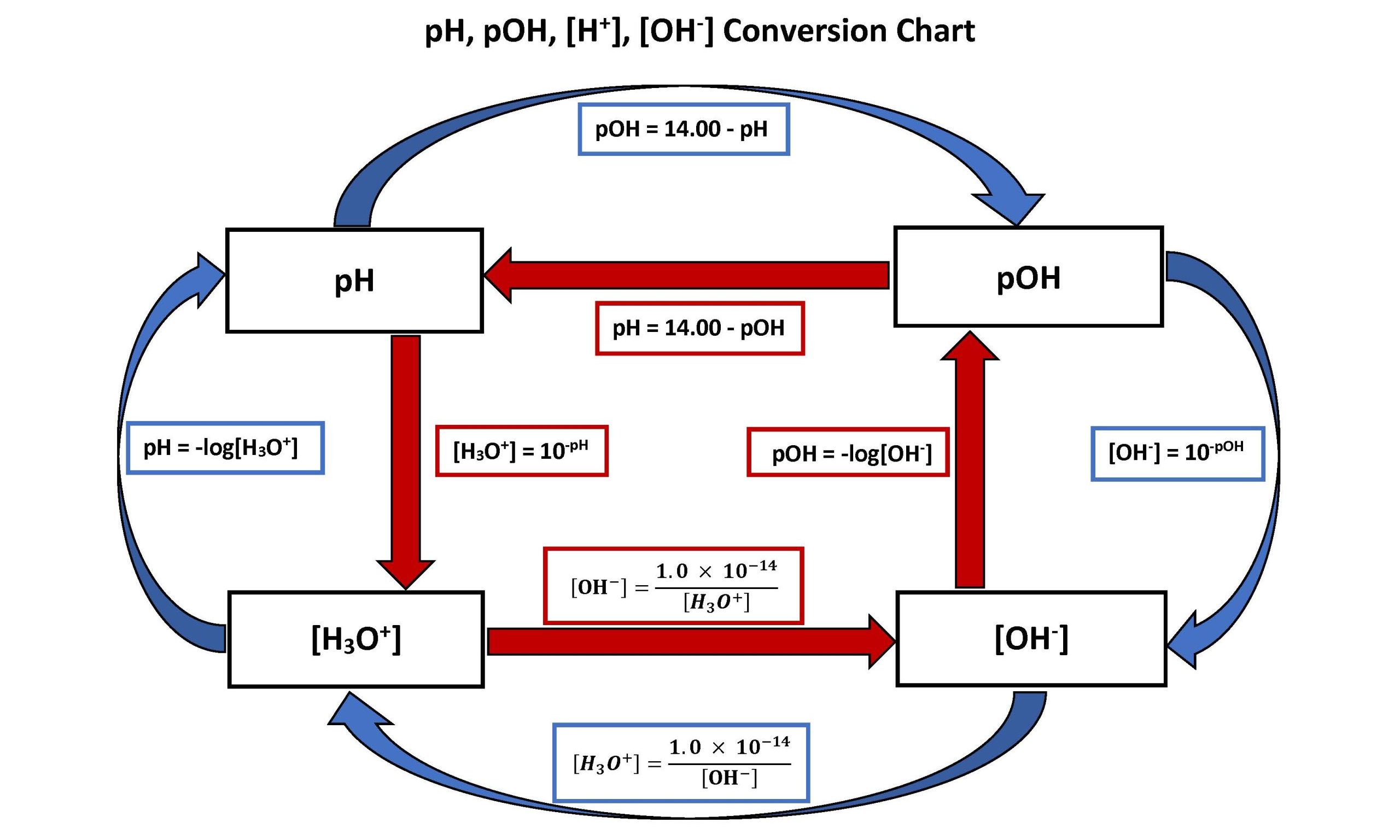
Understanding the Fundamentals: pH, [H+], and [OH-]
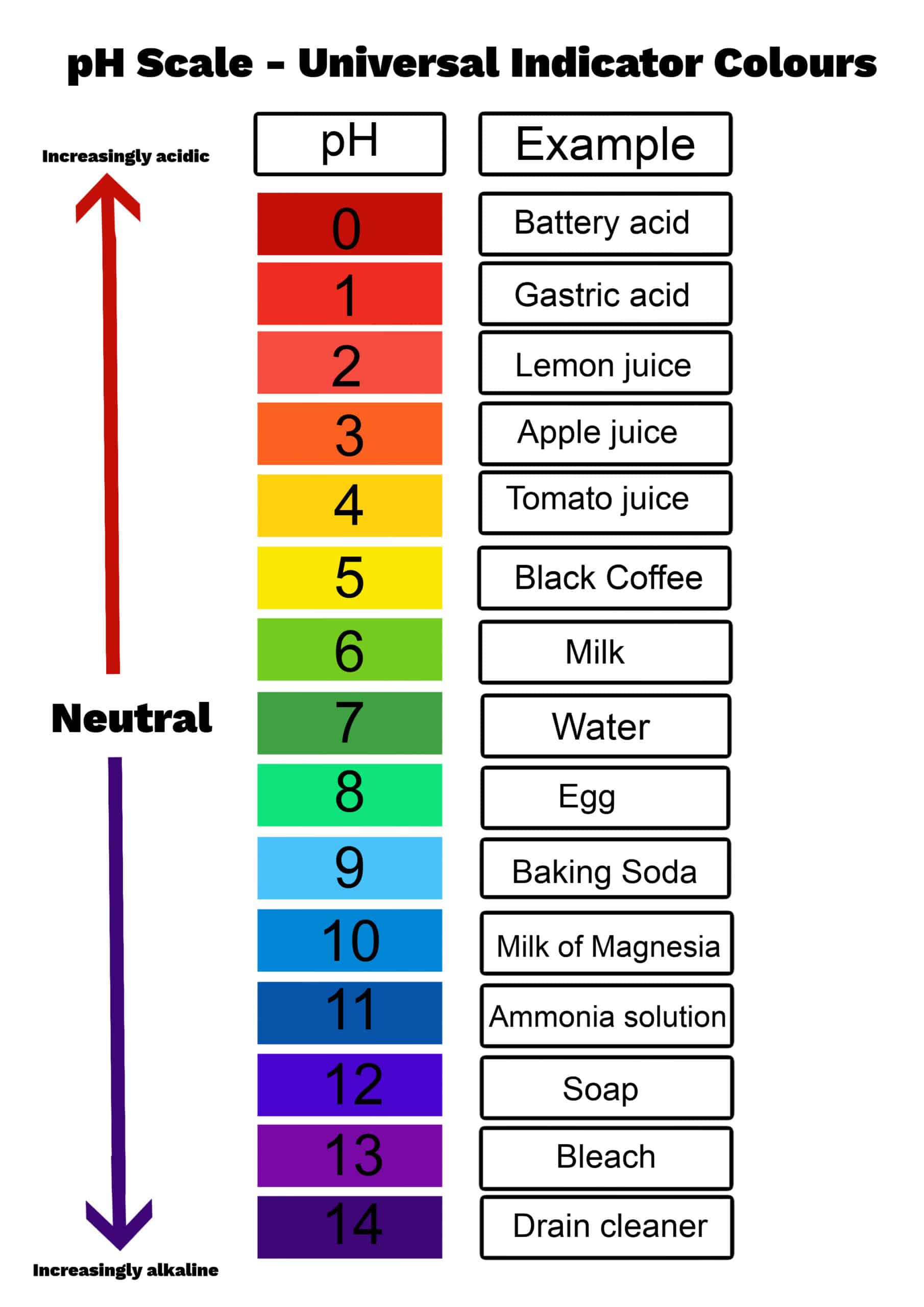
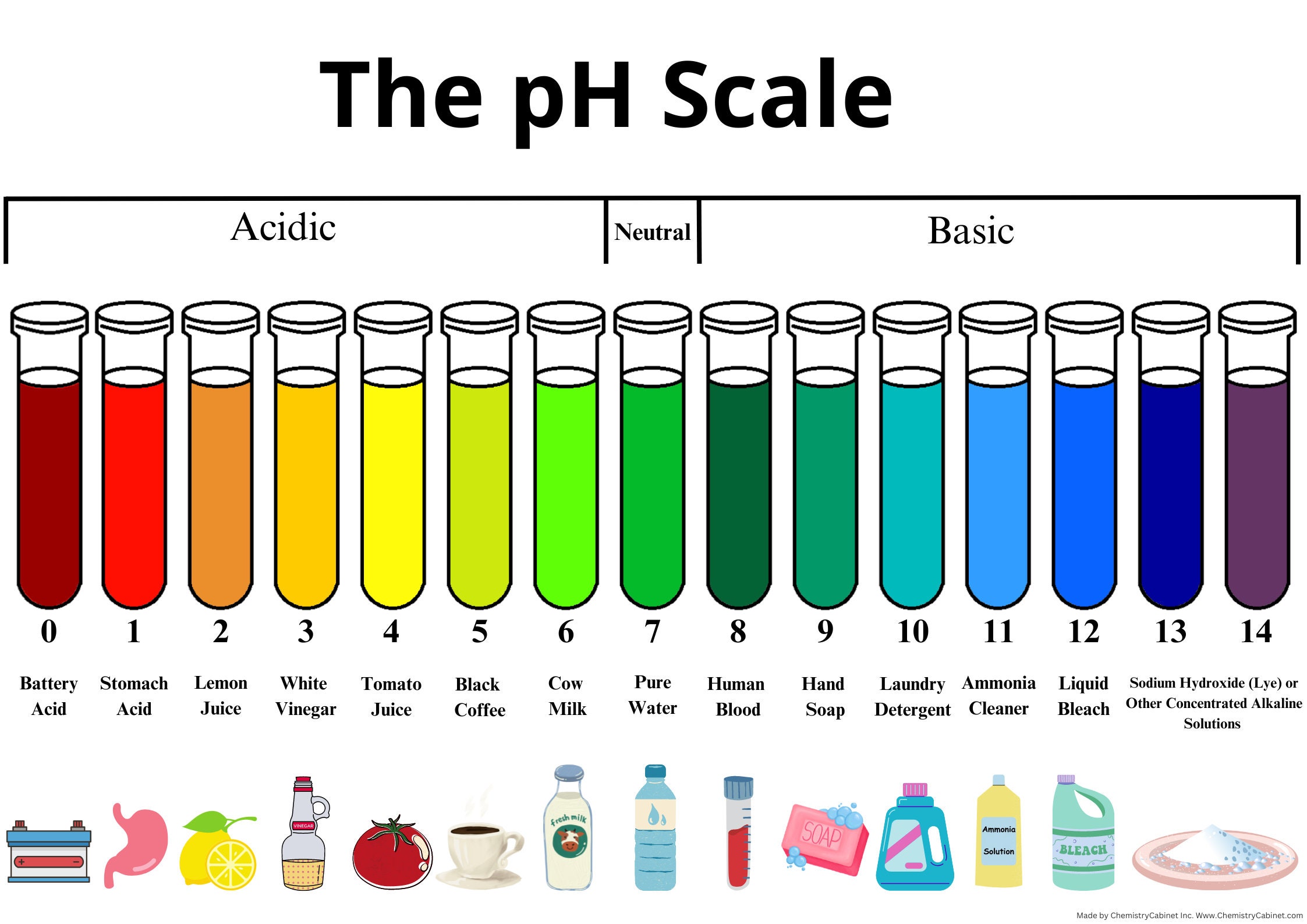
The pH scale ranges from 0 to 14, with 7 representing neutrality. Values under 7 point out acidity, whereas values above 7 point out alkalinity (or basicity). The dimensions is logarithmic, which means every entire quantity change represents a tenfold distinction in hydrogen ion focus. As an example, an answer with a pH of three is ten instances extra acidic than an answer with a pH of 4, and 100 instances extra acidic than an answer with a pH of 5.
The connection between pH and hydrogen ion focus is outlined by the next equation:
pH = -log₁₀[H+]
The place [H+] represents the focus of hydrogen ions in moles per liter (mol/L). Conversely, the hydrogen ion focus might be calculated from the pH utilizing:
[H+] = 10⁻ᵖᴴ
Equally, the hydroxide ion focus ([OH-]) is said to the pH by the ion product of water (Kw):
Kw = [H+][OH-] ≈ 1.0 x 10⁻¹⁴ at 25°C
Which means at 25°C, the product of [H+] and [OH-] is all the time fixed. Subsequently, figuring out the pH permits us to calculate each [H+] and [OH-]. A pH conversion chart usually presents these three parameters – pH, [H+], and [OH-] – in a tabular or graphical format, facilitating simple conversions and comparisons.
Establishing a pH Conversion Chart
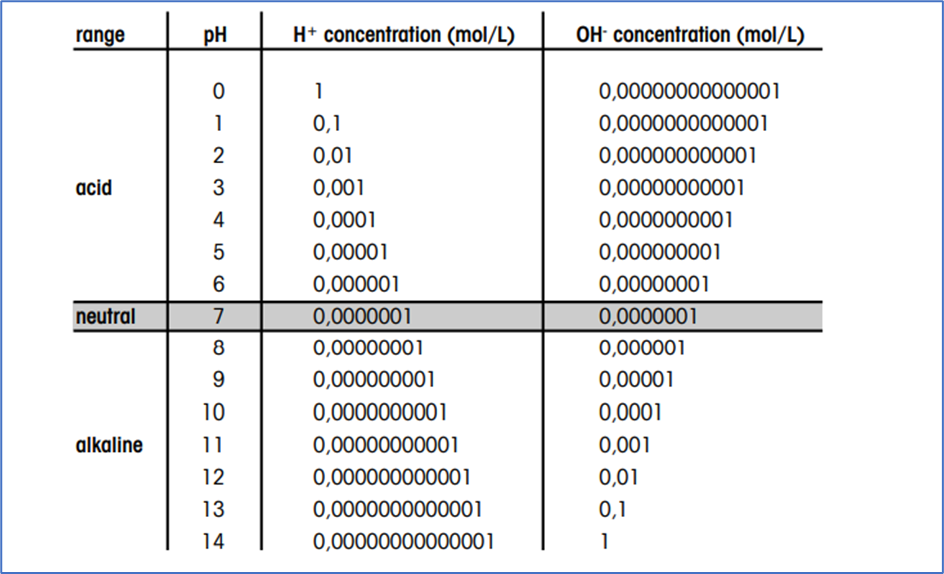
A fundamental pH conversion chart usually contains three columns: pH, [H+], and [OH-]. The pH column lists a variety of pH values, normally in increments of 0.1 or 1. The [H+] column is calculated utilizing the method [H+] = 10⁻ᵖᴴ, and the [OH-] column is calculated utilizing the method [OH-] = Kw/[H+], the place Kw is the ion product of water on the specified temperature (normally 25°C).
Instance of a Simplified pH Conversion Chart:
| pH | [H+] (mol/L) | [OH-] (mol/L) |
|---|---|---|
| 0 | 1 | 1 x 10⁻¹⁴ |
| 1 | 0.1 | 1 x 10⁻¹³ |
| 2 | 0.01 | 1 x 10⁻¹² |
| 3 | 0.001 | 1 x 10⁻¹¹ |
| 4 | 0.0001 | 1 x 10⁻¹⁰ |
| 5 | 0.00001 | 1 x 10⁻⁹ |
| 6 | 0.000001 | 1 x 10⁻⁸ |
| 7 | 0.0000001 | 0.0000001 |
| 8 | 0.00000001 | 0.000001 |
| 9 | 0.000000001 | 0.00001 |
| 10 | 0.0000000001 | 0.0001 |
| 11 | 0.00000000001 | 0.001 |
| 12 | 0.000000000001 | 0.01 |
| 13 | 0.0000000000001 | 0.1 |
| 14 | 0.00000000000001 | 1 |
Extra complete charts may embrace extra data, such because the corresponding pOH (calculated as pOH = 14 – pH at 25°C), or graphical representations to visualise the logarithmic relationship between the parameters. Some charts may also embrace color-coding to signify the acidity or alkalinity stage, making them extra user-friendly.
Purposes of pH Conversion Charts
pH conversion charts discover widespread use in varied fields:
-
Chemistry: In chemical laboratories, charts are important for understanding and controlling response circumstances. Many chemical reactions are pH-sensitive, and correct pH management is essential for reaching desired outcomes.
-
Biology: pH performs an important position in organic programs. The pH of bodily fluids, for instance, is tightly regulated to keep up homeostasis. Conversion charts assist biologists perceive the impression of pH adjustments on organic processes and organisms.
-
Agriculture: Soil pH considerably impacts nutrient availability to crops. Farmers use pH conversion charts to interpret soil take a look at outcomes and make knowledgeable selections about soil amendments to optimize crop yields.
-
Aquaculture: The pH of water in aquaculture programs should be fastidiously managed to make sure the well being and survival of aquatic organisms. Conversion charts assist aquaculture professionals monitor and modify water pH ranges.
-
Environmental Science: pH is an important indicator of water high quality. Conversion charts are used to evaluate the acidity of rainwater, lakes, and rivers, offering insights into environmental air pollution and its impression on ecosystems.
-
Meals and Beverage Business: The pH of meals merchandise is essential for meals security and preservation. Conversion charts are used to observe and management the pH of varied meals objects throughout processing and storage.
-
Drugs: pH performs a vital position in varied medical functions, together with blood evaluation and drug growth. Conversion charts are used to interpret blood pH ranges, diagnose acidosis and alkalosis, and optimize the effectiveness of medicines.
Limitations and Issues
Whereas pH conversion charts are invaluable instruments, it is important to acknowledge their limitations:
-
Temperature Dependence: The ion product of water (Kw) is temperature-dependent. Conversion charts primarily based on Kw at 25°C is probably not correct at different temperatures. For exact calculations at totally different temperatures, the suitable Kw worth should be used.
-
Exercise vs. Focus: The pH equation makes use of the focus of hydrogen ions. Nevertheless, in options with excessive ionic energy, the exercise of hydrogen ions (a measure of their efficient focus) differs from their precise focus. This discrepancy can have an effect on the accuracy of pH calculations, significantly in concentrated options.
-
Non-Aqueous Options: pH conversion charts are primarily relevant to aqueous options. The idea of pH is much less simple in non-aqueous solvents.
Conclusion
pH conversion charts are indispensable instruments for understanding and using the pH scale throughout varied scientific and industrial functions. By offering a transparent and concise illustration of the connection between pH, hydrogen ion focus, and hydroxide ion focus, these charts facilitate correct calculations, interpretation of information, and knowledgeable decision-making in numerous fields. Nevertheless, it is essential to concentrate on the restrictions of those charts and to make use of them appropriately, contemplating components corresponding to temperature and ionic energy. Understanding the elemental rules behind the pH scale and the development of conversion charts is crucial for his or her efficient and correct software.



Closure
Thus, we hope this text has offered invaluable insights into Decoding the pH Conversion Chart: A Complete Information. We thanks for taking the time to learn this text. See you in our subsequent article!