Decoding the Shapes of Molecules: A Complete Information to Molecular Geometry Charts
Associated Articles: Decoding the Shapes of Molecules: A Complete Information to Molecular Geometry Charts
Introduction
With enthusiasm, let’s navigate by means of the intriguing matter associated to Decoding the Shapes of Molecules: A Complete Information to Molecular Geometry Charts. Let’s weave attention-grabbing info and supply contemporary views to the readers.
Desk of Content material
Decoding the Shapes of Molecules: A Complete Information to Molecular Geometry Charts

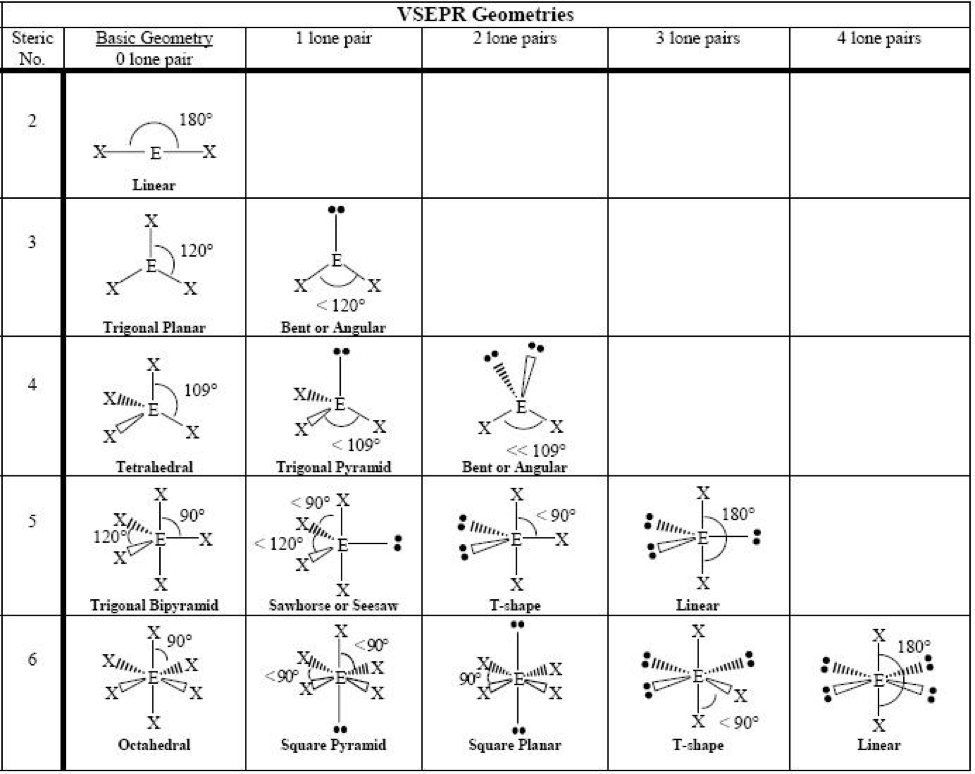
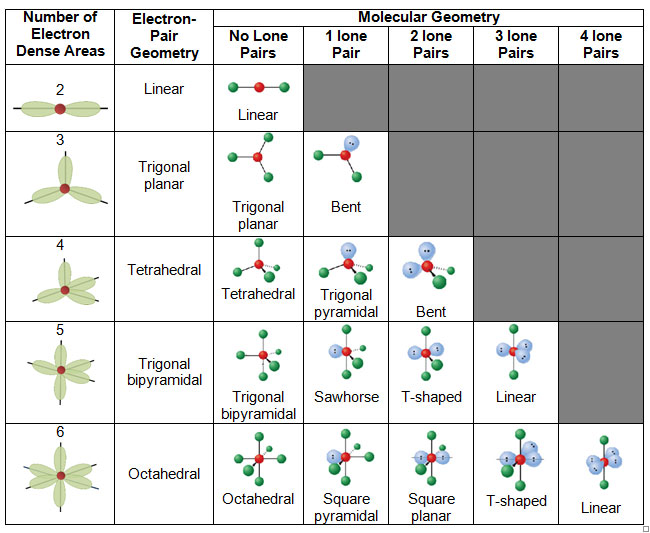
Molecular geometry, the three-dimensional association of atoms inside a molecule, is a cornerstone of chemistry. Understanding molecular geometry is essential for predicting a molecule’s properties, together with its reactivity, polarity, and bodily state. This intricate dance of atoms is ruled by the ideas of valence shell electron pair repulsion (VSEPR) principle, and its visualization is commonly aided by molecular geometry charts. This text delves into the intricacies of molecular geometry, explaining the VSEPR principle, exploring varied geometries, and offering an in depth interpretation of molecular geometry charts.
Understanding VSEPR Concept: The Basis of Molecular Geometry
The VSEPR principle posits that the electron pairs surrounding a central atom will prepare themselves to attenuate electrostatic repulsion. This minimization results in particular geometric preparations, dictating the general form of the molecule. The idea considers each bonding electron pairs (shared between atoms) and lone pairs (unshared electron pairs residing on the central atom). Each bonding and lone pairs affect the molecular geometry, however lone pairs exert a stronger repulsive drive as a result of their nearer proximity to the central atom.
The elemental steps in making use of VSEPR principle are:
-
Decide the Lewis construction: This entails drawing the Lewis dot construction, exhibiting all valence electrons and bonding preparations.
-
Depend the electron domains: An electron area is a area of electron density across the central atom. This consists of each bonding pairs and lone pairs.
-
Predict the electron area geometry: Primarily based on the variety of electron domains, the electron area geometry is predicted. Widespread electron area geometries embody linear (2 domains), trigonal planar (3 domains), tetrahedral (4 domains), trigonal bipyramidal (5 domains), and octahedral (6 domains).
-
Decide the molecular geometry: This step considers the association of atoms solely, disregarding the lone pairs. Lone pairs affect the bond angles and total form, however they aren’t included within the naming of the molecular geometry.
Widespread Molecular Geometries and Their Representations on Charts
Molecular geometry charts usually symbolize the completely different geometries utilizing varied notations and visible aids. Let’s discover some widespread geometries:
-
Linear: Two electron domains (two bonding pairs, no lone pairs). The atoms are organized in a straight line, with a bond angle of 180°. Examples embody BeCl₂ and CO₂. Charts typically symbolize this with a linear association of atoms.
-
Trigonal Planar: Three electron domains (three bonding pairs, no lone pairs). The atoms are organized in a flat triangle, with bond angles of 120°. Examples embody BF₃ and SO₃. Charts illustrate this as a flat triangle with the central atom on the heart.
-
Tetrahedral: 4 electron domains (4 bonding pairs, no lone pairs). The atoms are organized in a tetrahedron, with bond angles of roughly 109.5°. Examples embody CH₄ and SiCl₄. Charts often depict this as a three-dimensional tetrahedron.
-
Trigonal Pyramidal: 4 electron domains (three bonding pairs, one lone pair). The three bonding atoms kind a pyramid with the central atom on the apex. The bond angles are lower than 109.5° as a result of lone pair’s stronger repulsion. Examples embody NH₃ and PCl₃. Charts present a pyramidal construction with the lone pair implicitly understood.
-
Bent (V-shaped): 4 electron domains (two bonding pairs, two lone pairs). The 2 bonding atoms are organized in a bent or V-shape, with a bond angle lower than 109.5°. Examples embody H₂O and O₃. Charts depict this as a bent construction.
-
Trigonal Bipyramidal: 5 electron domains. This geometry is extra complicated, with atoms organized in a trigonal bipyramid. There are axial and equatorial positions, and the bond angles differ. Examples embody PCl₅ and SF₄. Charts typically use a three-dimensional illustration to make clear the axial and equatorial positions.
-
See-saw: 5 electron domains (4 bonding pairs, one lone pair). The lone pair occupies an equatorial place, distorting the geometry. Examples embody SF₄. Charts illustrate the distorted see-saw form.
-
T-shaped: 5 electron domains (three bonding pairs, two lone pairs). The 2 lone pairs occupy equatorial positions, resulting in a T-shaped molecule. Examples embody ClF₃. Charts present the attribute T-shape.
-
Linear (5 electron domains): 5 electron domains (two bonding pairs, three lone pairs). The 2 bonding pairs are organized linearly, with the lone pairs occupying the equatorial positions. Examples embody XeF₂. Charts present the linear association.
-
Octahedral: Six electron domains (six bonding pairs, no lone pairs). The atoms are organized on the corners of an octahedron, with bond angles of 90° and 180°. Examples embody SF₆ and [Fe(CN)₆]⁴⁻. Charts typically use a three-dimensional illustration to point out the octahedral construction.
-
Sq. Pyramidal: Six electron domains (5 bonding pairs, one lone pair). The lone pair occupies one of many positions, distorting the octahedron right into a sq. pyramid. Examples embody BrF₅. Charts present the pyramidal form with a sq. base.
-
Sq. Planar: Six electron domains (4 bonding pairs, two lone pairs). The 2 lone pairs occupy reverse positions, leading to a sq. planar geometry. Examples embody XeF₄. Charts depict the flat sq. association.
Deciphering Molecular Geometry Charts: Key Parts
Molecular geometry charts typically embody:
- Molecular Formulation: The chemical method of the molecule.
- Lewis Construction: The Lewis dot construction, exhibiting the association of atoms and electrons.
- Electron Area Geometry: The geometry primarily based on the whole variety of electron domains.
- Molecular Geometry: The geometry primarily based on the association of atoms solely.
- Bond Angles: Approximate bond angles between atoms.
- 3D Representations: Area-filling fashions or ball-and-stick fashions to visualise the three-dimensional construction.
- Polarity: Indication of whether or not the molecule is polar or nonpolar, primarily based on the symmetry and electronegativity variations between atoms.
Functions of Molecular Geometry Information
Understanding molecular geometry is essential in quite a few fields:
- Predicting Reactivity: The form of a molecule determines its accessibility to reactants, influencing its reactivity.
- Understanding Polarity: Molecular geometry dictates the distribution of cost, figuring out whether or not a molecule is polar or nonpolar, affecting its interactions with different molecules and its bodily properties.
- Spectroscopy: Molecular geometry influences the vibrational and rotational modes of molecules, that are noticed in spectroscopic methods like infrared (IR) and Raman spectroscopy.
- Drug Design: The form of a drug molecule is essential for its interplay with goal biomolecules, making molecular geometry a essential side of drug design.
- Supplies Science: The geometry of molecules influences the properties of supplies, reminiscent of their energy, conductivity, and reactivity.
Past the Fundamentals: Extra Advanced Situations
Whereas VSEPR principle offers a strong framework for understanding molecular geometry, some exceptions and complexities exist. Molecules with a number of central atoms, resonance constructions, and hypervalent compounds could require extra superior methods for correct geometry prediction. Computational strategies, reminiscent of density purposeful principle (DFT), are sometimes employed for such complicated instances.
Conclusion:
Molecular geometry charts function invaluable instruments for visualizing and understanding the three-dimensional preparations of atoms in molecules. By understanding VSEPR principle and decoding these charts successfully, chemists can predict molecular properties, perceive reactivity, and design new supplies and medicines. The data offered on this article offers a complete basis for decoding molecular geometry charts and making use of this information to varied chemical contexts. Additional exploration of superior methods and exceptions to VSEPR principle will improve one’s understanding of this basic side of chemistry.





Closure
Thus, we hope this text has offered beneficial insights into Decoding the Shapes of Molecules: A Complete Information to Molecular Geometry Charts. We hope you discover this text informative and helpful. See you in our subsequent article!