The Valency Chart of the Parts: A Complete Overview
Associated Articles: The Valency Chart of the Parts: A Complete Overview
Introduction
With enthusiasm, let’s navigate via the intriguing subject associated to The Valency Chart of the Parts: A Complete Overview. Let’s weave fascinating data and supply recent views to the readers.
Desk of Content material
The Valency Chart of the Parts: A Complete Overview
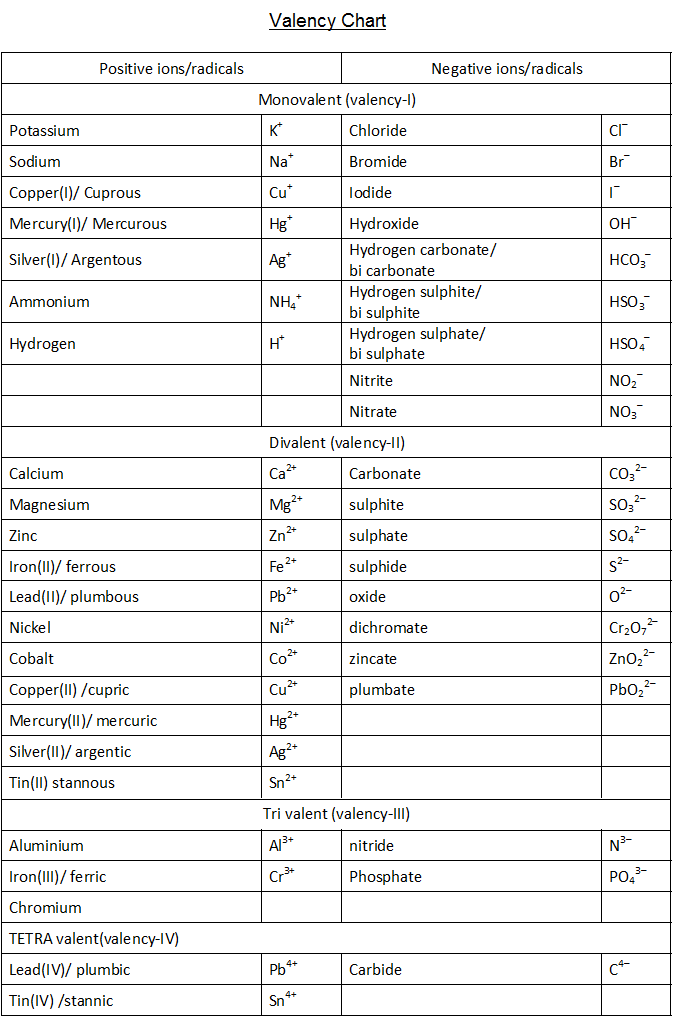
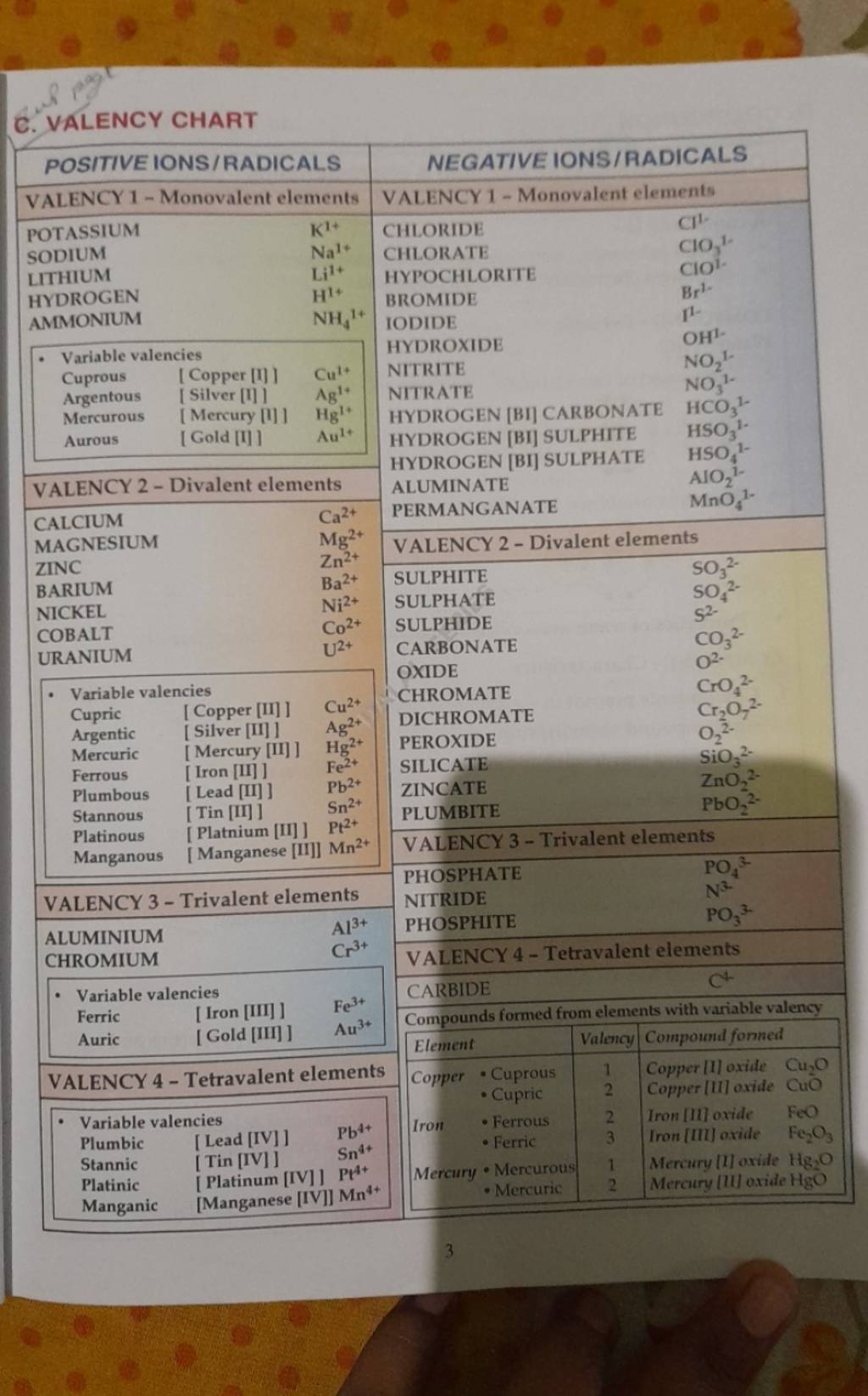
The idea of valency, or valence, is prime to understanding chemical bonding and the conduct of parts. It describes the combining capability of an atom, representing the variety of electrons an atom can achieve, lose, or share to attain a steady electron configuration, usually a full outermost electron shell. Whereas an entire, single valency chart encompassing all parts (together with artificial ones) with absolute precision is inconceivable because of the complexity of chemical conduct and the context-dependent nature of valency, we will discover a complete overview, categorized by teams and durations within the periodic desk, highlighting frequent valencies and exceptions.
Understanding Valency: Past a Easy Quantity
Valency is not merely a hard and fast quantity for every aspect. It may well differ relying on a number of elements:
- The aspect’s place within the periodic desk: Parts in the identical group usually exhibit comparable valencies on account of their comparable digital configurations.
- The kind of bonding: Valency can differ in ionic, covalent, and coordinate bonds. For example, a component would possibly exhibit the next valency in a covalent compound in comparison with an ionic one.
- The oxidation state: The oxidation state of a component displays the variety of electrons it has gained or misplaced (or seems to have gained or misplaced) in a compound. Whereas associated, valency and oxidation state aren’t at all times an identical. Oxidation states could be optimistic, unfavorable, or zero.
- The precise compound: The valency of a component can change relying on the opposite atoms it bonds with. Transition metals, specifically, are infamous for exhibiting a number of valencies.
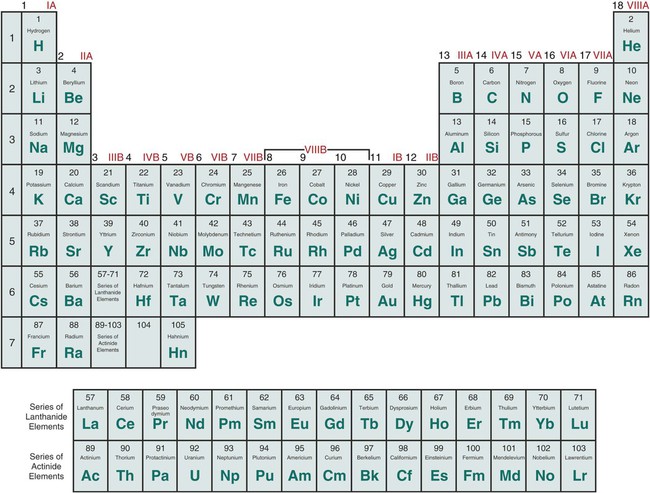
Valency and the Periodic Desk: A Group-wise Strategy
The periodic desk offers a robust framework for understanding valency traits. Let’s discover the frequent valencies for parts in several teams:
Group 1 (Alkali Metals): These parts (Li, Na, Okay, Rb, Cs, Fr) have one electron of their outermost shell. They readily lose this electron to kind a +1 ion, exhibiting a valency of 1. This constant valency makes them extremely reactive.
Group 2 (Alkaline Earth Metals): Be, Mg, Ca, Sr, Ba, and Ra possess two electrons of their outermost shell. They usually lose these two electrons to attain a steady configuration, leading to a valency of two. They’re much less reactive than alkali metals.
Group 13 (Boron Group): This group (B, Al, Ga, In, Tl) has three valence electrons. Whereas they’ll exhibit a valency of three, significantly in ionic compounds, their conduct is extra complicated. Boron, as an illustration, usually kinds covalent compounds with variable valency. Aluminum generally reveals a valency of three.
Group 14 (Carbon Group): C, Si, Ge, Sn, and Pb have 4 valence electrons. Carbon demonstrates a valency of 4, forming sturdy covalent bonds. Silicon and germanium additionally primarily exhibit a valency of 4, whereas tin and lead can present valencies of two and 4, relying on the response circumstances.
Group 15 (Pnictogens): N, P, As, Sb, and Bi have 5 valence electrons. They’ll achieve three electrons to attain a steady octet, leading to a -3 valency (e.g., NH₃). Nonetheless, they’ll additionally exhibit optimistic valencies, significantly in oxides and oxyacids (e.g., +3, +5).
Group 16 (Chalcogens): O, S, Se, Te, and Po possess six valence electrons. They usually achieve two electrons to attain a steady octet, resulting in a -2 valency (e.g., H₂O). Nonetheless, they’ll additionally exhibit optimistic valencies in compounds with extra electronegative parts (e.g., SO₃, the place sulfur has a +6 oxidation state).
Group 17 (Halogens): F, Cl, Br, I, and At have seven valence electrons. They readily achieve one electron to kind a -1 ion, exhibiting a valency of 1 (e.g., NaCl). Nonetheless, they’ll additionally kind covalent bonds, exhibiting optimistic oxidation states in compounds with extra electronegative parts like oxygen.
Group 18 (Noble Gases): He, Ne, Ar, Kr, Xe, and Rn have full outer electron shells (besides helium, which has a full shell with two electrons). They’re typically unreactive on account of their steady electron configuration, exhibiting a valency of 0. Nonetheless, heavier noble gases (like xenon) can kind compounds below particular circumstances, exhibiting optimistic valencies.
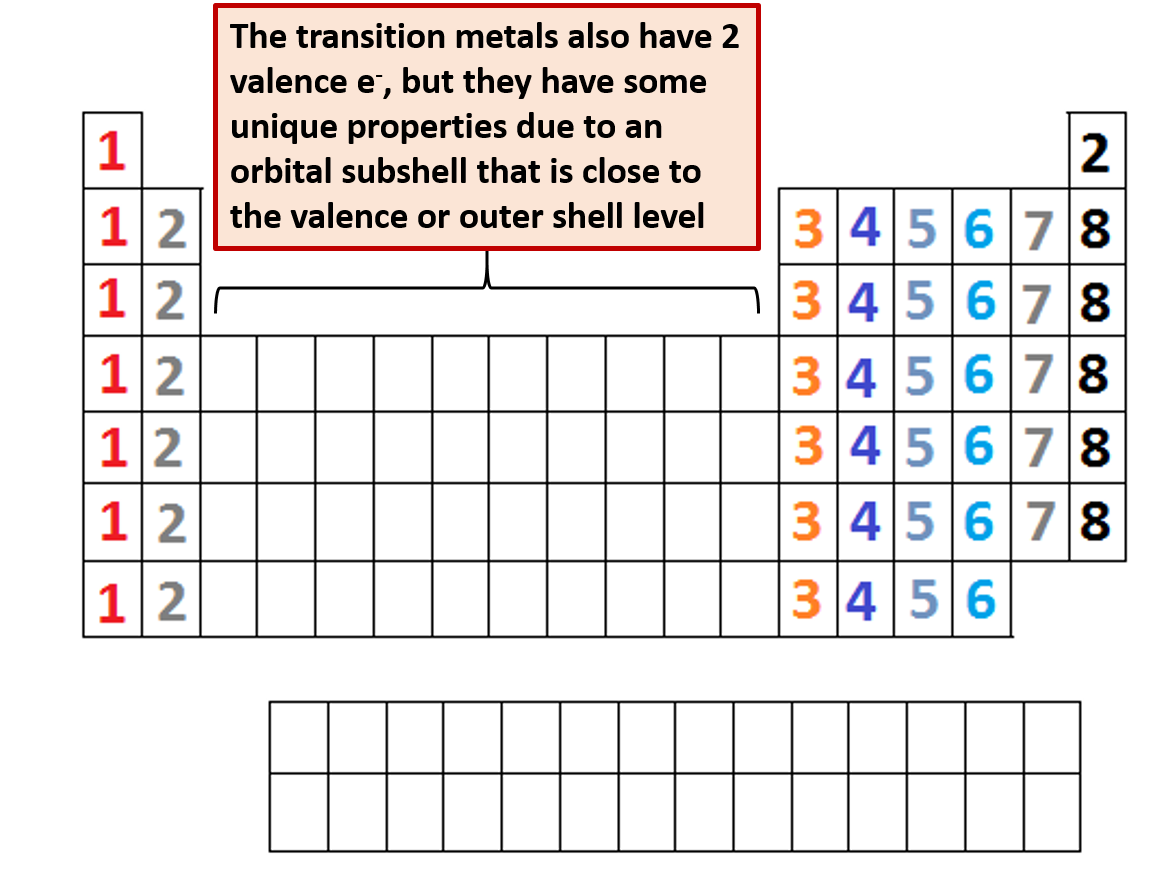
Transition Metals: The transition metals (positioned within the d-block) exhibit variable valencies. It is because they’ll lose electrons from each their s and d orbitals, resulting in a number of doable oxidation states and valencies. For instance, iron (Fe) can exhibit valencies of +2 and +3, forming compounds like FeO and Fe₂O₃. The valency of a transition metallic usually depends upon the ligands (atoms or teams bonded to the metallic) and the response circumstances.
Past the Foremost Teams: Lanthanides and Actinides
The lanthanides and actinides (f-block parts) additionally exhibit variable valencies, usually with +3 being a standard oxidation state. Nonetheless, they’ll present different oxidation states relying on the particular aspect and the response circumstances. Their chemistry is considerably extra complicated than that of the principle group parts.
Challenges in Making a Common Valency Chart
Creating a really complete valency chart for all parts faces a number of challenges:
- Context-dependent nature of valency: As mentioned, valency is not a hard and fast property; it depends upon the chemical surroundings.
- A number of valencies for a lot of parts: Particularly transition metals and a few predominant group parts show a number of valencies, making a easy chart unwieldy.
- Uncertainties for some parts: The chemistry of some less-studied parts, significantly artificial parts, stays incompletely understood.
- Complexity of bonding: The character of bonding (ionic, covalent, metallic, coordinate) considerably influences valency.
Conclusion:
Whereas a single, definitive valency chart for all parts is impractical because of the complexities outlined above, understanding the periodic traits and the elements influencing valency is essential for predicting and decoding chemical conduct. This text offers a framework for understanding frequent valencies and the exceptions that come up, emphasizing the significance of contemplating the particular chemical context when figuring out the valency of a component in a selected compound. Additional analysis into particular parts and their compounds is important for a extra exact understanding of their valency in several conditions. The periodic desk, with its inherent group and traits, stays probably the most priceless device in navigating the complexities of elemental valency.





Closure
Thus, we hope this text has supplied priceless insights into The Valency Chart of the Parts: A Complete Overview. We respect your consideration to our article. See you in our subsequent article!