Understanding and Using a pH Degree of Water Chart: A Complete Information
Associated Articles: Understanding and Using a pH Degree of Water Chart: A Complete Information
Introduction
With enthusiasm, let’s navigate by way of the intriguing subject associated to Understanding and Using a pH Degree of Water Chart: A Complete Information. Let’s weave fascinating data and provide recent views to the readers.
Desk of Content material
Understanding and Using a pH Degree of Water Chart: A Complete Information

Water, the elixir of life, is much extra complicated than its seemingly easy chemical system (H₂O) suggests. Its properties, and subsequently its suitability for varied purposes, are considerably influenced by its pH degree. Understanding the pH scale and the way it pertains to water high quality is essential for sustaining well being, guaranteeing environmental sustainability, and optimizing industrial processes. This text delves into the intricacies of water pH, gives an in depth pH degree of water chart, and explores its implications throughout numerous fields.
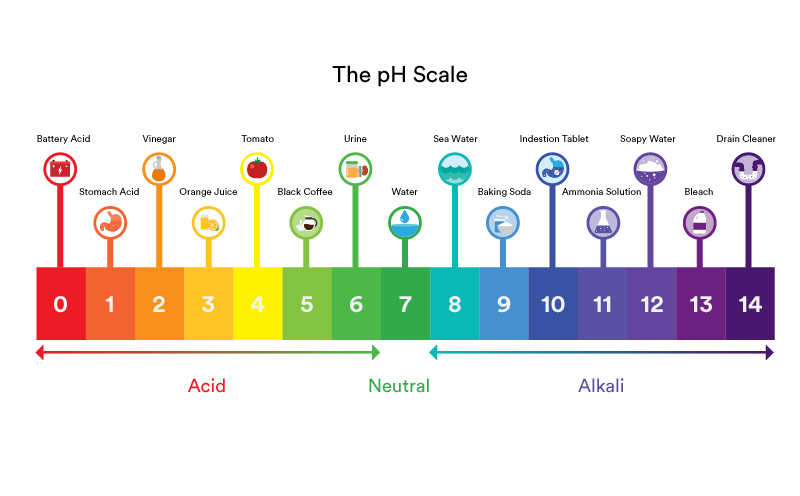
The pH Scale: A Measure of Acidity and Alkalinity
The pH scale is a logarithmic scale starting from 0 to 14, indicating the focus of hydrogen ions (H⁺) in an answer. A pH of seven is taken into account impartial, that means the focus of H⁺ ions is the same as the focus of hydroxide ions (OH⁻). Values under 7 point out acidity (larger focus of H⁺ ions), whereas values above 7 point out alkalinity (larger focus of OH⁻ ions). Every entire quantity change on the dimensions represents a tenfold change within the focus of H⁺ ions. As an illustration, an answer with a pH of 6 is ten occasions extra acidic than an answer with a pH of seven, and 100 occasions extra acidic than an answer with a pH of 8.
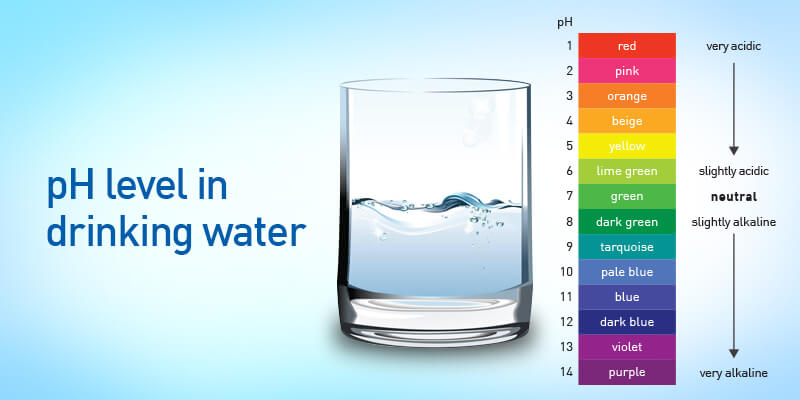
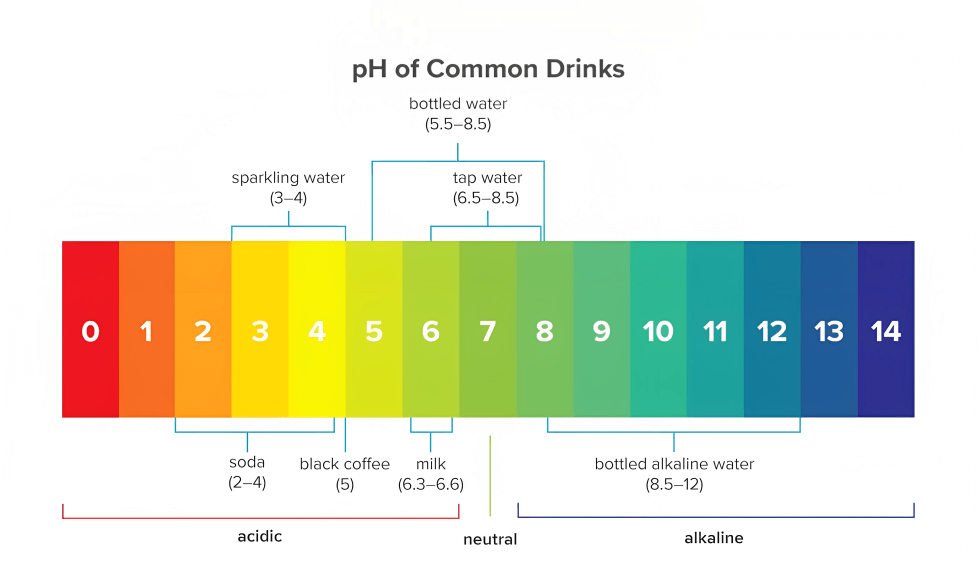
pH Degree of Water Chart: A Visible Illustration
The next chart gives an in depth overview of the pH ranges of water and their related traits:
| pH Degree | Description | Implications | Examples |
|---|---|---|---|
| 0-2 | Extraordinarily Acidic | Extremely corrosive, damaging to most supplies and dwelling organisms. | Industrial waste, sturdy acid spills |
| 3-4 | Very Acidic | Corrosive, dangerous to aquatic life, can leach metals from pipes. | Acid rain, some industrial effluents |
| 5-6 | Reasonably Acidic | May be corrosive, could have an effect on aquatic life and plant progress. | Barely acidic rain, some pure water sources |
| 7 | Impartial | Best for many dwelling organisms, minimal corrosiveness. | Pure, distilled water (ideally) |
| 8-9 | Barely Alkaline | Typically protected for many functions, could also be useful to some crops and organisms. | Some pure mineral waters, barely handled faucet water |
| 10-11 | Reasonably Alkaline | May be corrosive to some metals, could have an effect on the style of water. | Some pure mineral waters, water handled with lime |
| 12-14 | Very Alkaline | Extremely corrosive, damaging to many supplies and dwelling organisms. | Extremely alkaline industrial wastewater, some pure alkaline springs |
Components Affecting Water pH
A number of components affect the pH of water:
-
Pure Sources: The geological composition of the encircling soil and rocks considerably impacts water pH. Areas with excessive concentrations of minerals like limestone (calcium carbonate) are likely to have alkaline water, whereas areas with excessive concentrations of acidic minerals like sulfur may end up in acidic water.
-
Atmospheric Deposition: Acid rain, attributable to air air pollution, can decrease the pH of floor water our bodies.
-
Organic Exercise: The decomposition of natural matter can alter water pH. Bacterial exercise can produce acids, decreasing the pH, whereas the presence of sure algae can improve alkalinity.
-
Industrial Discharges: Industrial effluents usually include acids or bases that may drastically alter water pH.
-
Water Therapy Processes: Water remedy crops usually regulate the pH of water to optimize its potability and to forestall corrosion in pipes. This usually entails including chemical substances like lime (calcium hydroxide) to extend pH or acids to lower it.
Implications of Water pH Throughout Numerous Sectors
The pH of water has far-reaching implications throughout numerous sectors:
1. Human Well being: Whereas barely acidic or alkaline water is usually protected for consumption, extraordinarily excessive or low pH ranges will be dangerous. Extremely acidic water can corrode tooth enamel and irritate the digestive system. Extremely alkaline water may also trigger digestive issues. The perfect pH vary for ingesting water is usually thought of to be between 6.5 and eight.5.
2. Aquatic Ecosystems: Aquatic organisms are extremely delicate to modifications in water pH. Many species have slim pH tolerances, and important deviations from their optimum vary can result in stress, illness, and even loss of life. Acidification of water our bodies, usually attributable to acid rain, is a significant environmental concern, impacting fish populations and general ecosystem well being.
3. Agriculture: The pH of irrigation water can considerably affect plant progress. Totally different crops have completely different pH preferences; some thrive in barely acidic circumstances, whereas others choose barely alkaline circumstances. Sustaining the optimum pH vary for a specific crop is essential for maximizing yield and high quality.
4. Business: Water pH performs a crucial function in varied industrial processes. Sure industrial processes require particular pH ranges for optimum effectivity and to forestall corrosion of apparatus. For instance, the textile business makes use of water of particular pH ranges in dyeing and ending processes. The pH of boiler feedwater have to be rigorously managed to forestall scaling and corrosion.
5. Corrosion Management: The corrosiveness of water is instantly associated to its pH. Extremely acidic or alkaline water can corrode steel pipes and different infrastructure. Sustaining the suitable pH vary is essential for stopping corrosion and increasing the lifespan of water distribution techniques.
Measuring Water pH
Water pH will be measured utilizing varied strategies:
-
pH Meters: Digital pH meters are essentially the most correct technique for measuring pH. They use a sensor (electrode) that measures {the electrical} potential distinction between the answer and a reference electrode.
-
pH Take a look at Strips: These are cheap and handy for fast, approximate pH measurements. They modify shade relying on the pH of the answer.
-
pH Indicators: Chemical indicators, corresponding to litmus paper or common indicator, can be used to find out the approximate pH of water.
Conclusion:
Understanding the pH degree of water is crucial for a mess of purposes, from guaranteeing protected ingesting water to defending aquatic ecosystems and optimizing industrial processes. Using a pH degree of water chart, coupled with applicable measurement strategies, permits for knowledgeable decision-making and efficient administration of water assets. By rigorously monitoring and controlling water pH, we will safeguard human well being, shield the setting, and improve the effectivity of assorted industries. Continued analysis and technological developments in pH measurement and management will additional contribute to a extra sustainable and environment friendly use of this important useful resource.





Closure
Thus, we hope this text has supplied priceless insights into Understanding and Using a pH Degree of Water Chart: A Complete Information. We admire your consideration to our article. See you in our subsequent article!