Understanding and Using Solubility Charts in Pharmaceutical Improvement: An EP Perspective
Associated Articles: Understanding and Using Solubility Charts in Pharmaceutical Improvement: An EP Perspective
Introduction
With enthusiasm, let’s navigate by way of the intriguing subject associated to Understanding and Using Solubility Charts in Pharmaceutical Improvement: An EP Perspective. Let’s weave fascinating data and supply contemporary views to the readers.
Desk of Content material
Understanding and Using Solubility Charts in Pharmaceutical Improvement: An EP Perspective
Solubility, the power of a substance to dissolve in a solvent, is a cornerstone of pharmaceutical improvement. The European Pharmacopoeia (EP) would not explicitly current a single, complete "solubility chart," however quite incorporates solubility data and steerage all through its monographs and normal chapters. Understanding how solubility is addressed inside the EP framework is essential for guaranteeing the standard, security, and efficacy of pharmaceutical merchandise. This text delves into the significance of solubility in pharmaceutical formulation, explores how the EP handles solubility information, and discusses the sensible implications for pharmaceutical scientists.
The Significance of Solubility in Pharmaceutical Formulation
Solubility considerably impacts numerous features of drug improvement and manufacturing:
-
Bioavailability: For a drug to exert its therapeutic impact, it should first be dissolved within the organic fluids (e.g., gastrointestinal fluids, blood) to be absorbed into the systemic circulation. Poorly soluble medication exhibit low bioavailability, limiting their therapeutic efficacy.
-
Formulation Improvement: The solubility of an lively pharmaceutical ingredient (API) dictates the selection of formulation technique. Extremely soluble medication can typically be formulated into easy options or suspensions, whereas poorly soluble medication require extra subtle approaches, equivalent to strong dispersions, lipid-based formulations, or particle dimension discount strategies.
-
Stability: Solubility influences the soundness of the drug product. Modifications in solubility resulting from elements like pH, temperature, or the presence of excipients can result in precipitation, degradation, or crystallization, compromising the product’s high quality and shelf-life.
-
Drug Supply: The solubility profile of an API is a vital think about designing focused drug supply methods. Managed launch formulations typically depend on manipulating the solubility of the drug to realize the specified launch charge.
-
Analytical Strategies: Solubility information is crucial for growing and validating analytical strategies used to evaluate the standard and purity of drug substances and merchandise. As an illustration, solubility in several solvents is usually used to find out the purity of an API.
Solubility Descriptors within the European Pharmacopoeia
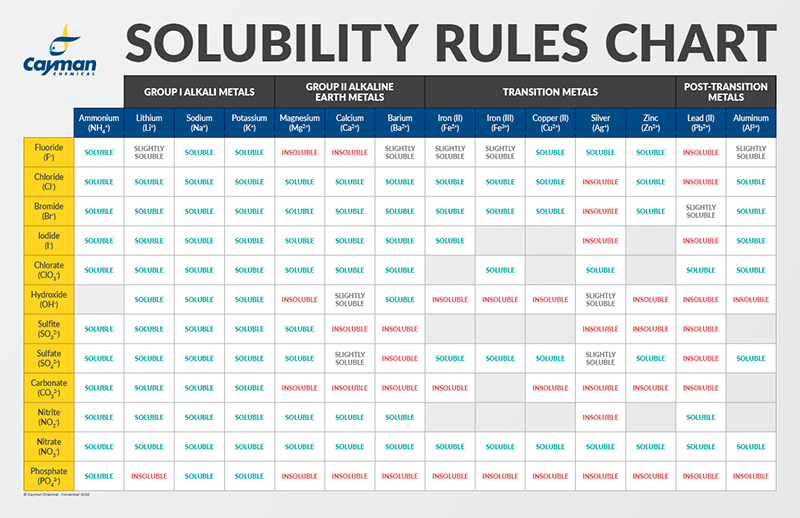
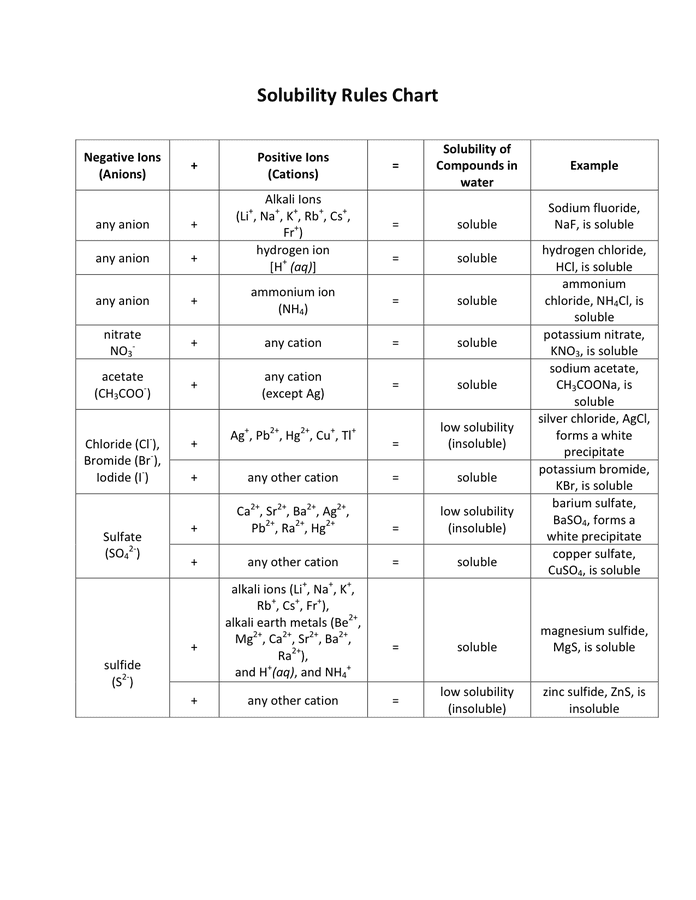
The EP would not present a single, universally relevant solubility chart. As a substitute, it employs a descriptive system primarily based on the quantity of solvent required to dissolve one a part of the solute. This qualitative description is usually ample for preliminary formulation improvement and high quality management. The system, detailed within the normal chapters of the EP, makes use of phrases like:
- Very soluble: Lower than 1 a part of solvent is required to dissolve 1 a part of solute.
- Freely soluble: From 1 to 10 elements of solvent are required.
- Soluble: From 10 to 30 elements of solvent are required.
- Sparingly soluble: From 30 to 100 elements of solvent are required.
- Barely soluble: From 100 to 1000 elements of solvent are required.
- Very barely soluble: From 1000 to 10,000 elements of solvent are required.
- Virtually insoluble: Greater than 10,000 elements of solvent are required.
These descriptors supply a normal indication of solubility, however they lack the precision wanted for detailed formulation design. The precise solubility values (e.g., expressed as mg/mL or g/L) are often decided experimentally and are sometimes included within the particular person monographs for particular APIs. These monographs may also specify the solvent used for the solubility willpower, as solubility is extremely solvent-dependent.
Figuring out Solubility: Experimental Strategies and EP Steering
The EP would not mandate a particular methodology for figuring out solubility, nevertheless it emphasizes the significance of utilizing validated and dependable strategies. Frequent strategies embrace:
-
Saturation shake-flask methodology: This includes equilibrating an extra of the solute with the solvent at a relentless temperature till saturation is achieved. The focus of the dissolved solute is then decided utilizing applicable analytical strategies (e.g., UV-Vis spectroscopy, HPLC).
-
HPLC methodology: Excessive-performance liquid chromatography (HPLC) is a robust approach for figuring out the solubility of APIs, even at very low concentrations. It gives excessive sensitivity and selectivity.
-
Spectroscopic strategies: UV-Vis and near-infrared (NIR) spectroscopy can be utilized to find out the focus of dissolved solute, offering a speedy and handy methodology for solubility willpower.
Whatever the methodology chosen, the EP emphasizes the significance of rigorously controlling parameters equivalent to temperature, pH, and the presence of excipients, as these elements can considerably affect solubility. The monograph for every API might present particular steerage on the suitable methodology and circumstances for solubility willpower.
Solubility Enhancement Strategies and EP Issues
For poorly soluble medication, numerous strategies are employed to reinforce their solubility and bioavailability. These strategies embrace:
-
Salt formation: Changing the API right into a salt kind can considerably enhance its solubility. The EP typically consists of data on appropriate salt varieties for particular APIs.
-
Co-solvents: Including co-solvents (e.g., ethanol, propylene glycol) to the formulation can improve the solubility of the API. The EP offers steerage on the appropriate ranges of co-solvents in pharmaceutical merchandise.
-
Stable dispersions: Dispersing the API in a water-soluble provider (e.g., polyvinylpyrrolidone) can improve its dissolution charge and solubility.
-
Particle dimension discount: Lowering the particle dimension of the API will increase its floor space, resulting in improved dissolution charge and solubility. The EP might specify particle dimension limits for sure formulations.
-
Complexation: Forming complexes between the API and a complexing agent can enhance solubility.
When utilizing these strategies, it is essential to make sure that the chosen methodology complies with the EP necessities concerning security, efficacy, and stability. The number of excipients and the formulation course of should be rigorously managed to ensure the standard and security of the ultimate product.
The Position of Solubility in High quality Management
Solubility performs a vital position in high quality management testing. The EP monographs typically specify solubility limits for APIs and completed merchandise to make sure consistency and high quality. Deviation from these limits might point out issues with the manufacturing course of or the soundness of the product. Solubility testing is due to this fact a necessary a part of the standard management procedures for pharmaceutical merchandise.
Conclusion
Whereas the EP would not present a single solubility chart, it gives complete steerage on solubility willpower and its significance in pharmaceutical improvement. Understanding the EP’s method to solubility, together with its descriptive system and the emphasis on validated experimental strategies, is essential for pharmaceutical scientists. The cautious consideration of solubility all through the drug improvement course of, from API choice to formulation design and high quality management, is crucial for guaranteeing the security and efficacy of pharmaceutical merchandise. The knowledge offered in particular person monographs, mixed with an intensive understanding of the final chapters on solubility and associated matters, offers the mandatory framework for navigating this vital side of pharmaceutical science. Moreover, steady analysis and improvement in solubility enhancement strategies are important for bettering the bioavailability and therapeutic efficacy of poorly soluble medication, a unbroken problem within the pharmaceutical trade.





Closure
Thus, we hope this text has offered precious insights into Understanding and Using Solubility Charts in Pharmaceutical Improvement: An EP Perspective. We thanks for taking the time to learn this text. See you in our subsequent article!