Understanding Valency: A Complete Information for Ninth Graders
Associated Articles: Understanding Valency: A Complete Information for Ninth Graders
Introduction
On this auspicious event, we’re delighted to delve into the intriguing subject associated to Understanding Valency: A Complete Information for Ninth Graders. Let’s weave fascinating data and supply recent views to the readers.
Desk of Content material
Understanding Valency: A Complete Information for Ninth Graders
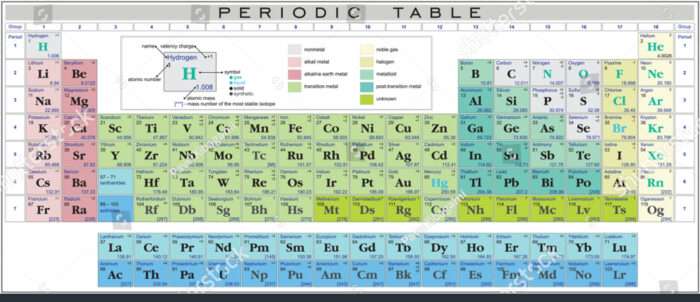
Chemistry, at its core, is the research of matter and its interactions. One essential side of understanding these interactions is greedy the idea of valency. Valency dictates how readily an atom of a component combines with different atoms to kind chemical compounds. This text will delve into the idea of valency, clarify learn how to interpret a valency chart, and supply an in depth have a look at the valency of varied parts generally encountered in Ninth-grade chemistry.
What’s Valency?
Valency, merely put, is the combining capability of an atom. It represents the variety of electrons an atom can achieve, lose, or share to attain a steady electron configuration, usually resembling that of a noble fuel (Group 18 parts). Noble gases have a full outermost electron shell (valence shell), making them exceptionally steady and unreactive. Atoms of different parts attempt to attain this steady configuration by chemical bonding.
This drive in direction of stability is the driving pressure behind chemical reactions. Atoms with incomplete valence shells will react with different atoms to both achieve, lose, or share electrons to fill their valence shells. The variety of electrons an atom positive aspects, loses, or shares is its valency.
Varieties of Valency:
Whereas the definition of valency is easy, the best way atoms obtain stability results in various kinds of valency:
-
Electrovalency (or Ionic Valency): This happens when atoms switch electrons to attain a steady configuration. One atom loses electrons (changing into a positively charged ion or cation), and one other atom positive aspects these electrons (changing into a negatively charged ion or anion). The magnitude of the cost on the ion represents the electrovalency. For instance, sodium (Na) loses one electron to change into Na⁺ (electrovalency of +1), whereas chlorine (Cl) positive aspects one electron to change into Cl⁻ (electrovalency of -1).
-
Covalency: This entails the sharing of electrons between atoms to attain a steady configuration. Atoms share electrons to finish their outermost shells. The variety of electron pairs shared represents the covalency. For instance, in a methane molecule (CH₄), carbon shares 4 electron pairs with 4 hydrogen atoms, leading to a covalency of 4 for carbon and 1 for every hydrogen atom.
-
Coordinate Covalency (or Dative Covalency): It is a particular kind of covalency the place each electrons within the shared pair are contributed by the identical atom. This typically happens when one atom has a lone pair of electrons and one other atom has an empty orbital. For instance, within the ammonium ion (NH₄⁺), nitrogen donates a lone pair of electrons to kind a coordinate covalent bond with a hydrogen ion (H⁺).
Understanding the Valency Chart:
A valency chart is a desk that lists the weather and their respective valencies. These charts are essential for predicting the formulation of chemical compounds. The valency of a component is usually represented by a Roman numeral (e.g., I, II, III, IV) or an Arabic numeral. It is vital to notice that some parts can exhibit variable valency, that means they will have totally different valencies relying on the response situations or the opposite parts they’re bonding with.
Valency Chart (Simplified):
Whereas an entire valency chart could be in depth, we will create a simplified chart for frequent parts encountered in Ninth-grade chemistry:
| Ingredient | Image | Valency |
|---|---|---|
| Hydrogen | H | I |
| Lithium | Li | I |
| Sodium | Na | I |
| Potassium | Okay | I |
| Magnesium | Mg | II |
| Calcium | Ca | II |
| Beryllium | Be | II |
| Aluminium | Al | III |
| Carbon | C | IV |
| Silicon | Si | IV |
| Nitrogen | N | III, V |
| Phosphorus | P | III, V |
| Oxygen | O | II |
| Sulphur | S | II, IV, VI |
| Chlorine | Cl | I |
| Bromine | Br | I |
| Iodine | I | I |
| Fluorine | F | I |
Necessary Issues:
-
Variable Valency: Discover that some parts, like nitrogen, phosphorus, and sulfur, exhibit variable valency. This implies they will kind compounds with totally different valencies relying on the response situations and the opposite atoms concerned. That is as a result of availability of various vitality ranges and the potential of forming a number of bonds.
-
Group Developments: Observe the periodic desk. Components throughout the similar group (vertical column) typically have related valencies. For instance, Group 1 parts (alkali metals) usually have a valency of +1, whereas Group 2 parts (alkaline earth metals) often have a valency of +2. Understanding periodic tendencies helps predict valency.
-
Predicting Chemical Formulation: The valencies of parts are essential for figuring out the chemical formulation of compounds. The system should be electrically impartial; the full optimistic cost should equal the full unfavourable cost. For instance, to kind a compound between sodium (Na, valency +1) and chlorine (Cl, valency -1), the system is NaCl (one sodium ion for each chloride ion). For magnesium (Mg, valency +2) and chlorine (Cl, valency -1), the system is MgCl₂ (one magnesium ion for each two chloride ions).
Examples and Purposes:
Let’s illustrate the appliance of valency in figuring out chemical formulation:
-
Water (H₂O): Hydrogen has a valency of I, and oxygen has a valency of II. To stability the valencies, two hydrogen atoms mix with one oxygen atom.
-
Carbon Dioxide (CO₂): Carbon has a valency of IV, and oxygen has a valency of II. To stability, one carbon atom combines with two oxygen atoms.
-
Aluminium Oxide (Al₂O₃): Aluminium has a valency of III, and oxygen has a valency of II. To realize neutrality, two aluminium atoms (whole optimistic cost of +6) mix with three oxygen atoms (whole unfavourable cost of -6).
-
Ammonium Chloride (NH₄Cl): Ammonium (NH₄⁺) has a valency of +1 (as a result of +1 cost), and chlorine has a valency of -1. Due to this fact, the system is NH₄Cl.
Conclusion:
Understanding valency is key to greedy the rules of chemical bonding and predicting the formulation of chemical compounds. This text has offered a basis for Ninth-grade college students to grasp the idea of valency, various kinds of valency, learn how to interpret a valency chart, and learn how to use valency to foretell chemical formulation. Additional exploration of the periodic desk and its tendencies will solidify this understanding and supply a powerful foundation for extra superior chemistry ideas. Bear in mind to follow utilizing the valency chart to foretell formulation and analyze the chemical bonding in numerous compounds. This can improve your understanding and problem-solving abilities in chemistry. By mastering this elementary idea, you may be well-equipped to deal with extra advanced chemical ideas in your future research.








Closure
Thus, we hope this text has offered worthwhile insights into Understanding Valency: A Complete Information for Ninth Graders. We hope you discover this text informative and useful. See you in our subsequent article!